Cáncer de cabeza y cuello experiencia en Guatemala con cetuximab
Induction chemotherapy followed by radiotherapy plus cetuximab for patients with laryngeal cancer (LC), Guatemala Experience

Phase II trial:
induction chemotherapy followed by radiotherapy conventional plus
cetuximab weekly for patients with squamous-cell carcinoma locally
advanced of laryngeal cancer, treated at the Instituto Guatemalteco de
Seguridad Social (IGSS), Guatemala, Guatemala.
Castro H, Hernandez C, García L, Castro N, Salazar L
Patients with unresectable, locally
advanced stage III and IVA tumors of laryngeal cancer have satisfactory
5-years OS rate with induction chemotherapy (TAX 323, TAX 324), and
cetuximab in combination with radiotherapy improved locoregional control
and overall survival in patients with locally advanced tumors of the
head and neck. The interpretation of the results of the
cetuximab-radiotherapy trial is quite difficult, since chemotherapy was
not part of the study, we conducting a small phase II study treated at a
single institution with chemoradiotherapy plus cetuximab, with the
following schema: induction therapy with cisplatin (75 mg/m2) and
docetaxel (75 mg/m2) every 3 weeks for four cycles followed cetuximab
day 1 of radiotherapy with a loading dose of 400 mg per square meter,
followed by 250 mg per square meter weekly throughout radiotherapy.
Results: from march-2009 to
jun-2011 13 patients previously untreated, locally advanced squamous-
cell carcinoma of laryngeal, have been included in order to identify
patterns of recurrence, toxic effects, and SLD. Median age was 59 (23 –
84). 62% patients present with stage III and 38% stage IVA. Whit a
median follow-up of 22 months, the actuarial 2-years DFS is 62%. Local
disease relapse occurred in 5 patients (38%). The most common grade 3
or 4 adverse events were neutropenia (32% and 18%), 23% had grade 3 skin
reactions. There were no related deaths, all but one of the patients
with recurrence died of cancer. Significant prognostic factors for
recurrence by univariate analysis were age (<60 vs >60) (p=0.03).
The 2-year overall survival for laryngeal cancer was 80%.
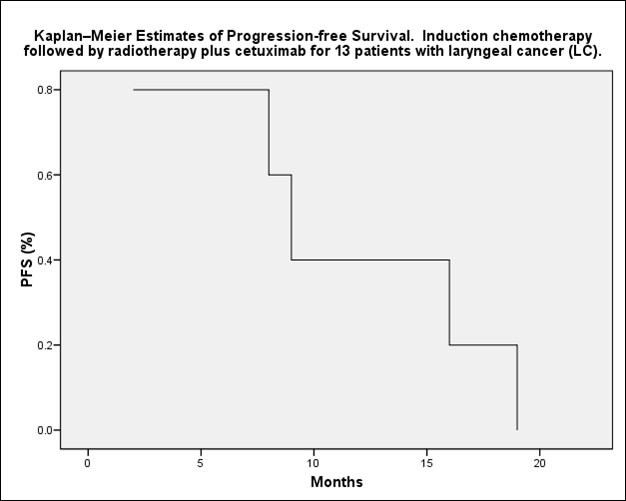
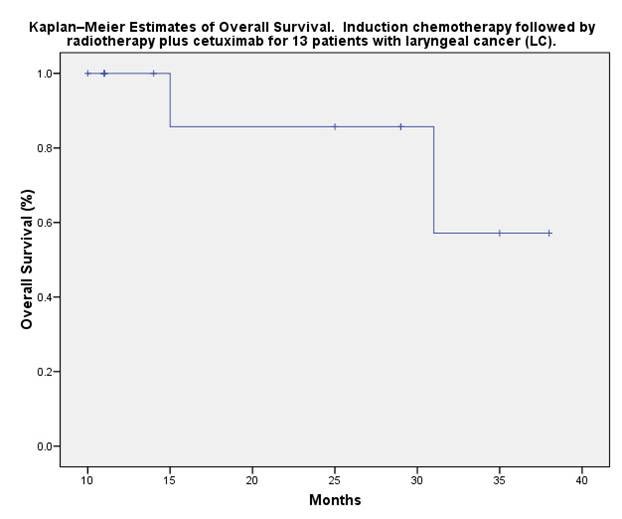
Conclusions: induction
chemotherapy followed by radiotherapy with cetuximab for patients with
advanced laryngeal cancer is feasible and safely, these preliminary
results indicate that requires confirmation from randomized trials.
Related publications
- Angeles Medical Group, English Version
- Chemotherapy and side effects
- Clinical Significance of WHO Classification and Cell Kinetic Study using PCNA and Ki-67 on Thymic Epithelial Neoplasms
- Clinical Trials
- CRECE english version
- Hepatocarcinoma en Guatemala
- Molecular zone, clinical laboratory
- Our services
- Slides and presentations
- Studies in Guatemala
- Types of cancer
- Video conferences
- Who we are