Clinical Significance of WHO Classification and Cell Kinetic Study using PCNA and Ki-67 on Thymic Epithelial Neoplasms
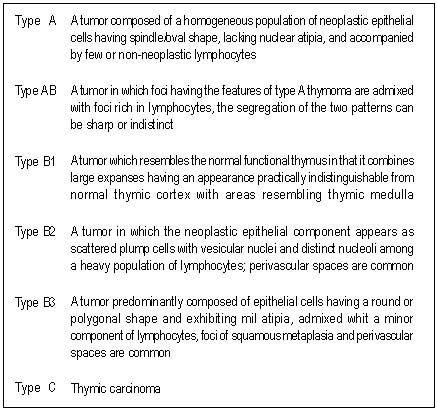 |
| Table 1. The definition of WHO classification of thymic epithelial tumors |
Condensed abstract
Cell kinetic study to evaluate if there is a correlation
between proliferation indices(PI) of the neoplastic thymic cells and
histological type with stage and OS.
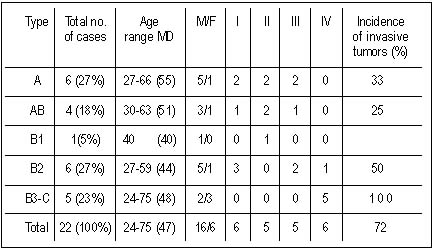 |
| Table 2. Clinical stage and the incidence of invasive tumors according to WHO classification |
Abstract Purpose
Thymic epithelial neoplasms(TEN) are rare tumors in
which the importance of histology is controversial. We performed cell
kinetic study to evaluate if there is a correlation between
proliferation indices(PI) of the neoplastic cells and histological type
with stage and OS.
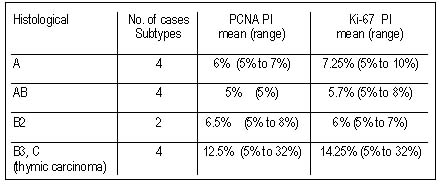 |
| Table 3. Correlation between PCNA and Ki-67 labeling indices and histological subtypes |
Patients and methods
Retrospective study of patients diagnosed with
TEN(1988–2000). Demographic and clinical variables and complete
follow-up data were obtained. PI using PCNA and Ki-67 were analyzed.
Results. We studied 22 patients. Median age was 47 years(range
24-75). Histological classification: type A=6(27%), AB=4(18%),
B1=1(4.5%), B2=6(27%), B3-C(thymic carcinoma)=5(23%). Invasive tumors
were seen in 72.72% of cases. Although the mean values of PCNA and Ki-67
were higher in types B3-C(12.5% and 14.25%) than in types A-B1-B2(5.8%
and 6.1%), there was no association with histological type. An
association among histologic type (A-AB-B2 vs B3-C) and stage (I-II-III
vs IV; p=0.001) was found. A complete resection was achieved in 74% of
cases. Complete remission was achieved in 18/21 patients who received
definitive treatment. There were 6(28.6%) perioperative deaths and 3
patients relapsed. Five-year DFS for patients with B3-C was 40% compared
with 100% for other subtypes. The actuarial 5-year OS of patients with
B3-C was 80%, whereas that of patients with other types was 88%.
Stage(p=0.001) and histology B3-C(p=0.008) were the only significant
variables in predicting recurrence. Stage IV (p=0.005) and age>
60years (p=0.03) increased the risk of death.
Discussion. The clinical behavior of TEN depends on stage and
histology. There was no correlation between PI and stage or histology. A
larger study is warranted.
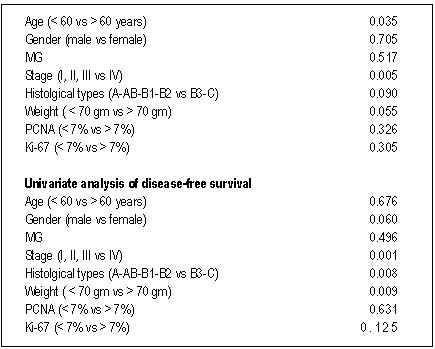 |
| Table 4. Univariate analysis of survival |
Introduction
Several classification systems for thymic epithelial
neoplasm (TEN) are currently in use.1-5 The low incidence of these
neoplasms and the lack of uniformity in the reported series made
necessary the unification of criteria thus resulting in the World Health
Organization (WHO) classification.6 A critical issue is the confuse
nomenclature of these classifications and an absence of consensus
regarding their prognostic value. Nowadays the most important predictive
factors for outcome still are complete resection7 and stage according
to Masaoka classification8 (I: totally encapsulated, II: invasion
through capsule, III: invasion into organs -pericardium, lung, great
vessels-, IVa: pleural or pericardial implants and IVb: hematogenous
metastasis).
Efforts have been made to discover immunohistochemical markers that
identify factors of poor prognosis9-11. Few studies have attempted to
correlate the proliferation index (PI) with the histological type12.
Cellular cycle is complex and some proteins are expressed exclusively in
the phases in which they intervene and their presence can reflect the
PI of the cell. Monoclonal antibodies directed against them have been
developed allowing to study two groups of proteins: those acting on DNA
synthesis during S-phase like PCNA (proliferating cell nuclear
antigen)13 and those expressed in proliferating but not resting cells
like Ki-6714,15. These methods have shown that in some neoplasms high
proliferative rates correlate with poor prognosis. These data correlate
with those obtained by flow cytometry16.
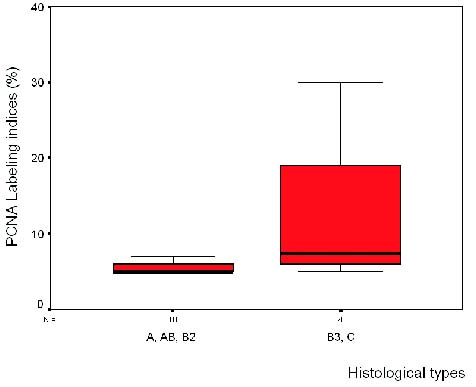 |
| Figure 1. PCNA labeling indices vs histological types. There was no difference between the other histoloical types |
Patients and Methods
Patients. Patients diagnosed with TEN in our institution
between 1988 and 2000 were included in the analysis. Age, gender,
clinical presentation, association with MG, stage, treatment, and
complete follow-up date were obtained from clinical records.
Histological classification. Analysis of tissue samples and
classification according to the WHO classification was carried out by a
pathologist (AG) in a blinded fashion. TEN were divided in four groups
depending on their nuclei characteristics (type A: spindle/oval shape,
type B: dendritic or epitheloid appearance). Tumors combining both
morphologies were designated as type AB, and type C (atypia and
necrosis). Type B tumors were further subdivided into three categories
(B1, B2 and B3) on the basis of the proportional increase in the
epithelial component and cytologic atypia. (Table 1)
Immunohistochemistry. Studies were performed on paraffin embedded
tissue sections in 14 cases using monoclonal antibodies directed against
PCNA and Ki-67 (Dako, Carpinterra, CA) in an automated immunostaining
equipment (Ventana, Nexes, Tucson, AZ) as suggested by the manufacturer.
Labeling index was determined by light microscopy with an oil-immersion
objective randomly counting 500 tumor epithelial cells and expressing
the results as a percentage of positive cells.
Statistical analysis. Cross-tabulation between present status and
clinical variables (age, gender, presence of MG, tumor weight, invasion,
stage, histological type and PI using PCNA and Ki-67) were used to
screen for significant associations using the Chi-square test for
independence. The correlation between PCNA and Ki-67 labeling indices
was carried out using the Spearman correlation test. Overall survival
(OS) and disease-free survival (DFS) curves were plotted according to
the method described by Kaplan and Meier and analyzed using the log-rank
test. A p value was considered significant if it was below 0.05. The
analysis was performed using the SPSS-10 commercial software package.
Results
Clinical presentation and diagnostic methods. Twenty-six patients
were diagnosed with TEN between 1988 and 2000. Four were excluded
because of incomplete data. Sixteen patients (72%) male and 6(28%)
female were included. Male-to-female ratio was 2.6:1. Mean age was 50.5
years (range 24-75). Sixteen patients (72%) had symptoms of MG at onset,
2(9%) presented with symptoms attributable to anterior mediastinal mass
(i.e. shortness of breath and chest pain), 1 patient (4.5%) had
disautonomia at onset and in 1(4.5%) the diagnosis was preceded 12
months by pure red blood cell aplasia. The tumor was discovered on
routine chest radiograph in 2(9%) patients. No other paraneoplastic
syndromes were found. According to Masaoka classification there were 6
patients in stage I, 5 in stage II, 5 in stage III and 6 in stage IV.
Histological diagnosis was made in material obtained using fine-needle
aspiration (4.5%), core biopsies (9%) or surgical resection (86.5%).
According to WHO classification 27% were type A, 18% AB, 4.5% B1, 27%
B2, and 23% B3-C types (Table 2).
The computed tomography (CT) was abnormal in all cases, suggesting an
invasion that was proved by histological studies in 16 of them (72%
sensitivity and 100% specificity). When invasive TEN were analyzed we
found necrosis in 9/16 patients (56%; p<0.05) and mediastinal
lymphadenopathy in 11/16 patients (69%; p<0.05).
Mean tumor weight was 70 grams (range 30–100) and mean diameter was
8.5 cm (range 3.5–15.1). There were no differences between invasive and
no invasive TEN in weight (< 70 vs. > 70 gr.; p=0.09) or diameter
(< 8.5 vs. > 8.5 cm.; p=0.6).
Determination of PCNA and Ki-67 labeling indices. Although mean
values of both PI were higher in types B3-C (stage IV) as compared to
types A-AB-B2 (Table 3 and figures 1 and 2), there was no association
between levels of PCNA and Ki-67 (<7% vs. > 7%) and histological
type (A-AB-B2 vs B3-C; p=0.099; p=0.48 respectively). The patients who
relapsed showed higher PI (mean PCNA=12.5%; mean Ki-67=14.25%) than
those who did not relapsed (PCNA=5.83% and Ki-67=6.31%), although the
difference was not statistically significant. The Spearman’s correlation
coefficient between PCNA and Ki-67 was 0.389. An association among
histological type (A- AB-B2 vs B3-C) and stage (I-II-III vs IV)
(p=0.001) was found.
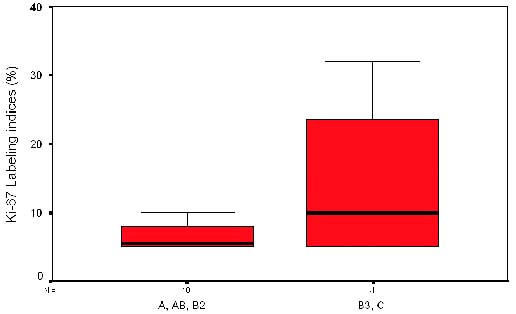 |
| Figure 2. Ki-67 labeling indices vs histological types. Thymic carcinoma showed higher labeling indices by ki-67 antibody compared with the A-AB-B2 but there were no significant differences. |
Treatment and results
Complete resection was achieved in 16(74%) patients,
partial resection in 5(23%) and 1(4.5%) was solely biopsed. The approach
was medial sternotomy in 19 cases (86.71%) and in 2 cases (9.52%) it
was posterolateral thoracotomy. Ten (45%) patients required resection of
pericardium or lung. In this series 16(72%) patients presented with
symptoms of MG. After definitive resection 8 (50%) patients remained
asymptomatic, 7(44%) had partial improvement and 1(6%) did not respond. A
patient develop gastric non-Hodgkin’s lymphoma 4 years after the
thymoma diagnosis.
The mean follow-up was 58 months (range 12–228). Six patients (27%)
received postoperative radiotherapy in doses varying from 40 to 60 Gy
(complete resection=1 and partial resection=5). Of these 3(13.5%) had
stage III (type A=1 and B2=2) TEN, and 3(13.5%) had stage IV thymic
carcinoma. One patient did not accept treatment (stage IVa, B2) and is
alive 24 months after diagnosis. Of the 21 patients who received
definitive treatment, 18 achieved complete remission. Of these 12
(54.5%) were alive without relapse at last follow-up. There were
3(13.5%) perioperative deaths (2 stage II and 1 stage IV) due to
myasthenic crisis, 3(13.6%) patients died of unrelated causes. Three
patients (13.5%) relapsed during the follow-up period (B3=2, C=1) at 41,
61 and 24 months respectively. The relapse site was lung in 2 patients
and retroperitoneum in 1. In all cases complete resection was possible.
Two patients are disease-free at 12 and 18 months and the other
developed a new recurrence 5 months after resection and is currently
being treated with chemotherapy.
The recurrence probability of TEN was 51% at 24 months and 40% at 60
months. When A-AB-B2 and B3-C groups were compared, 5-year DFS was 100%
and 40% respectively. On univariate analysis only stage (I, II, III vs
IV; p=0.001) and histological classification (A, AB, B1, B2 vs B3 and C;
p=0.008) were of value for the prediction of recurrence(Figure 3).
The actuarial 5-year survival rate for the entire group was 86%. For
patients with type B3-C it was 80% at 5 year, whereas for patients with
other types it was 88%. On univariate analysis both stage IV (p=0.005)
and age > 60 years (p=0.03) were risk factors for death(Table 4).
Histology (A-B1-B2 vs. B3-C) was correlated with stage (I-II-III vs IV,
p=0.001). The actuarial 5-year survival rate according to Masaoka
classification was: stage I=100%(6/6), II=60%(3/5), III=100%(5/5) and
IV=83.3% (5/6)(Figure 4).
Discussion
Higher absolute values of PCNA and Ki-67 expression was
found in types B3-C than in types A, AB, B1, B2 TEN, thus reflecting a
low growth proliferation in non-invasive or minimally invasive thymic
epithelial neoplasias, although no statistically significant differences
were observed.
In the reported series of TEN male and female patients16 are equally
affected and 70% of cases occur during the sixth and seventh decades of
life (mean age 53)17. In our series we found a slight male
predominance(male-to-female ratio 2.6:1) and patients were younger(mean
age 47). We also found a high prevalence of associated systemic
syndromes and incidental finding was made only in 9% of our patients in
contrast to other published data in which this occurs in 40 to 50% of
patients18.
Imaging studies play an important role in the detection and staging of
TEN. CT is the method of choice in the evaluation of a mediastinal mass
with a 72% sensitivity and 100% specificity for invasive TEN. CT can
differentiate cystic from solid lesions and the presence of fat or
calcium within the lesion. The findings suggestive of invasion include
necrosis and mediastinal lymphadenopathy as well as obliteration of
mediastinal fat planes or poor demarcation of tumor from adjacent
structures. Baron et al19 showed that in patients with proven thymomas,
the CT showed an ovoid soft-tissue density mass in the anterior
mediastinum and necrosis in 100% of patients. An important consideration
to be made is whether the lesion determines the treatment plan.
Prognostic factors that have been associated with better survival
include non-invasion, lower stage and complete surgical resection.
Invasion is reported in 30% to 40% of the patients8. Bernatz et al.18
demonstrated an overall 15-year survival rate of 12.5% in the invasive
group and 47% in the non invasive group. Several studies have examined
the effect of the extent of surgical resection on OS and DFS. In 241
operative cases, Maggi and colleagues20 found an 82% OS rate in those
patients whose tumors underwent complete resection and 26% survival rate
at 7 years in those undergoing biopsy alone. Yagui et al21 reported
excellent long-term survival with extended resection in patients with
stages III–IV. Their overall 5- and 10-year survival rates were 77% and
59%, respectively. Ten out of 12 patients who underwent resection of the
superior vena cava had a long term survival without evidence of
recurrence. Nakahara and coworkers22 have shown that the survival rate
in patients with stage III disease undergoing complete resection was
comparable to those patients with stage I and II disease. Therefore,
regardless of stage, tumor resection is one of the import predictors of
treatment outcome. The Masaoka stage is also a prominent prognostic
factor8. Stages I-II can be considered together as a group with a
favorable prognosis; stages III-IV have a significantly worse prognosis.
In general, complete resection rate is about 70%, in nearly all stage
I-II and in 27%-44% of stage III patients14. In this series the complete
resection rate was 71% (stage I-II: 100%, stage III-IV: 40%) which does
not differ from published data.
Most TEN are slow-growing tumors and at diagnosis they present with a
high tumor volume, the mean diameter in this series was of 8.5 cms.
Caruso et al24 reported a mean diameter of 9.9 cm. Some authors suggest
that recurrence rate seem to be related to the size and stage of TEN. No
recurrence was observed if the tumor was less than 5 cm in diameter and
a recurrence rate of 15% and 33% was observed if the tumor diameter was
5–15 cm and more than 15 cm. resepectively25. In our series this fact
could not be confirmed but perhaps this is due to the number of patients
studied.
When TEN was suspected and the preoperative evaluation suggested a
resectable tumor, the surgical approach was medial sternotomy (86%),
which provides a wide exposure of the anterior mediastinum26. Previous
reports yielded an approximate 25% of invasive tumors.
In our series we found 22.3% non invasive and 72.7% invasive tumors.
These percentages were higher than reported because carcinomas (22.72%
in our series) were included in the analysis. Difference between these
data and previously reported data is due to the fact that in previous
reports carcinomas were not included. Using the WHO classification
Okumura et al reported 53.6% of invasive tumors (12.14% of which were
carcinomas)27. These data does not differ to much from ours.
The TEN are biologically heterogeneous tumors as it is demonstrated
by their association with paraneoplastic syndromes28. We found a higher
than expected prevalence of MG (72%), probably because our institution
is a national referral center for complex diseases. Improvement in
myasthenic symptoms is almost always recognized following thymectomy,
and complete remission rates varies from 7% to 63%1. In our series it
was 50%. The prevalence of association with MG in reference to Masaoka
classification was higher among stages I-II (100%) than among stages III
(60%) and IV (66%), but no significant difference in survival was noted
between patients with invasive and noninvasive thymomas.
Currently, death after TEN resection in the perioperative period is
rare and should be less than 6%29. In this series 3 deaths (13.6%)
occurred, all of them in patients with poorly controlled MG and
respiratory complications. Modern preoperative preparation, intensive
care facilities and plasmapheresis have reduced this risk.
Verley and Hollman2, Maggi and colleagues20 and Lewis and co-workers3
did not find a significant difference in the prognosis of patients with
and without MG. In the present study patients without MG had a higher
survival rate (100%) than patients with MG (84%), although difference
was not significant. The presence of MG may even confer a survival
advantage, but this may be due to a predominance of incidentally
discovered early-stage tumors in myasthenic patients.
Some authors consider the use of radiotherapy in all patients after
either a complete or partial surgical resection and even as primary
treatment29. In this series 27.3% received postoperative radiotherapy
with adequate local control. In 10/11 cases in stages III-IV, complete
remission was achieved without adjuvant therapy. These data demonstrate
that the most important factor predicting outcome is the complete
resection of the tumor. In patients who have residual macroscopic
disease, radiation therapy achieves local control in 60% to 90% of cases
regardless the stage. Three (13.6%) patients had distant relapses,
similar to the reported 9-11%30. The actuarial 5-year survival rate was
86% in our series.
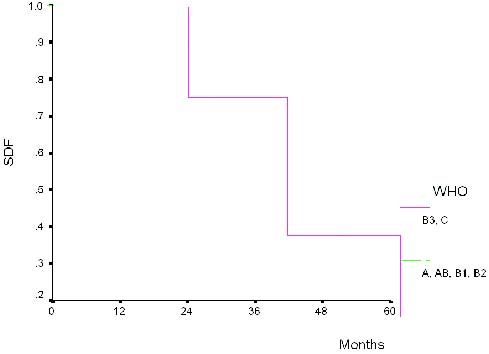 |
| Figure 3. Disease-free survival for patients with types A-AB-B1-B2 and B3-C |
Our results differ from those reported in regard to age,
male-to-female ratio, association with MG, proportion of thymic
carcinomas and OS rate. These differences cannot be explained only by a
selection bias and they could show great variability in cell
composition, histologic grow patterns and different biological behavior
in our population. However, since this is the only study carried out in
our country, we cannot fully support this notion.
We examined the clinical significance of histologic classification of
TEN proposed by WHO. We analyzed separately the group of malignant
thymoma and thymic carcinoma. Seventy-seven percent of cases were types
A-AB-B1-B2. The higher proportion corresponded to types A and B2 (35.2%)
and the less frequent was the B1 (4.55%). MG was associated to type
A-AB-B1-B2 in 66.6%, 75%, 100% and 100% respectively. We found thymic
carcinoma in 23% of cases, all in stage IV. The mean age in these
patients was 48 years, similar to previously reported31. We did not
found correlation between stage and invasion with PI.
Although TEN have been traditionally separated into benign and
malignant categories, a reliable distinction cannot be made on the basis
of either histopathologic or electron microscopic findings. Malignancy
can only be demonstrated by the finding of invasion of the tumor capsule
or surrounding organs, or by the presence of metastasis31.
The traditional classification based on invasiveness, number of
lymphocytes and epithelial cell architecture is descriptive but
straightforward and based on numerous clinicopathologic studies. The
most consistent prognostic factor is the presence of invasion through
the capsule1-3. In our series we found correlation between DFS and stage
with WHO classification only in cases of thymic carcinoma. This fact
could be explained because of the aggressive clinical course of this
disease.
Few studies have correlated the proliferative index with the
histological type. Chilosi and colleagues32 examined the proportion of
proliferation in 8 cases of thymoma. Using the Ki-67 antigen they found a
large increase in the activity of all examined samples ranging from 35%
to 80% and the expression of this antigen was much higher than that
seen in age matched control thymuses.
These authors speculated whether if this phenomenon might explain the
pathogenesis of autoimmune diseases in these patients. Woo-Ick et al13
found a correlation between PCNA and Ki-67 IP (Spearman coefficient =
0.72). We could not confirm this finding. In summary analysis of a
larger group of patients will be required to determine whether
proliferation fraction as determined by this method can predict outcome.
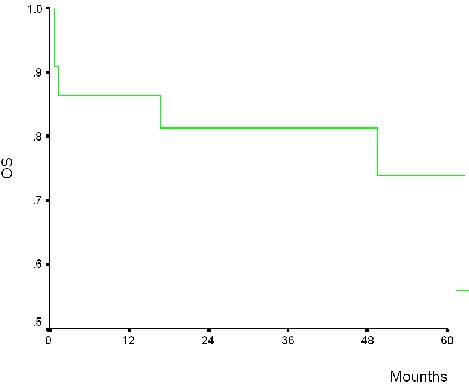 |
| Figure 4. Actuarial overall survival and disease-free survival for entire group of 22 thymic epithelial neoplasm |
Address. Dr. Hugo Raúl Castro Salguero. Grupo Ángeles,
2da calle 25-19 zona 15, Vista Hermosa I, Edificio Multimédica, oficina
10-15. Telefax: (502) 23857572. E-mail. hugoraulcastro@hotmail.com
Running title. Cell kinetics in thymic epithelial neoplasms
Key words: Thymic epithelial neoplasms, proliferation indices, histologic type, WHO classification
References
1. Loehrer P. Thymomas: current experience and future direction in therapy. Drugs 45(4):477-487, 1993
2. Verley JM, Hallman KH. Thymoma: a comparative study of clinical
features, histologic features and survival in 200 cases. Cancer
55:1074-1086, 1985
3. Lewis RJ, Wick MR, Scheithaur BW, Taylor WF. Thymoma. A clinicopathologic review. Cancer 60:2727-2743, 1987
4. Marino M, Müller-Hemerlink HK. Thymoma and thymic carcinoma: relation
of thymoma epithelial cells to the cortical and medullary
differentiation of thymus. Virchows Arch 407:119-149, 1985
5. Suster S, Moran CA. Thymoma, atypical thymoma, and thymic carcinoma: a
novel conceptual approach to the classification of thymic epithelial
neoplasms. Am J Clin Pathol 111:826-833, 1999
6. Rosai J, Sobin LH. Histological typing of tumors of the thymus,
International Histologic Classification of tumors, 2nd ed. New York,
Berlin: Springer, 1999, pp 1 – 25
7. Loehrer P. Current approaches to the treatment of thymoma. Ann Med 31:73-9, 1999 (suppl 2)
8. Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study fo
thymomas with special reference to their clinic stage. Cancer
48:2485-2492, 1981
9. Shimizu J, Hayashi Y, et al. Primary thymic carcinoma: a
clinicopathological and immunohistochemical study. J Surg Oncol
56(3):159-64, 1994
10. Ring NP, Addis BJ. Thymoma: an integrated clinicopathological and immunohitochemical study. J Pathol 149(4):327-37, 1986
11. Pich A, Chiarle R, Chiusa L, et al. Argyrophilic nucleolar organizer
region counts predict survival in thymoma. Cancer 74:1568-1574, 1994
12. Pich A, Chiarle R, Chiusa L, et al. Long-term survival of thymoma
patients by histologic patients by histologic pattern and proliferative
activity. Am Surg Pathol 19:918-926, 1995
13. Yang W, Efird J, Quintanilla L, Harris N. Cell kinetic study of
thymic epithelial tumors using PCNA (PC10) and Ki-67 (MIB-1) antibodies.
Human Pathol 27(1):70-76, 1996
14. Pan CC; Ho DM; Chen WY; Huang CW; Chiang H. Ki-67 labelling index
correlates with stage and histology but no significantly with prognosis
in thymoma. Histopatology 33(5):453-8, 1998
15. Gilhus NE; Jones M; Turley H; Gatter KC; Nagvekar N; et al. Oncogene
protein and proliferation antigens in thymomas: increased expression of
epidermal growth factor receptor and Ki-67 antigen. J Clin Pathol
48(5):447-55, 1995
16. Hainsworth J, Greco A. Mediastinal tumors in Haskell M (edd) Cancer
treatment. Philadelphia, W.B. Saunders Company, 2001 pp 652 – 657
17. Lanfenfeld J, Graeber GM. Current management of thymoma. Surg Oncol Clin N Am 8(2):327-39, 1999
18. Bernatz PE, Khonsari S, Harrison EG, et al. Thymoma: factors incluencing prognosis. Surg Clin North Am 53:885-893, 1973
19. Baron R, Joseph K, Sagel S, Levitt R. Computed tomography of the abnormal thymus. Radiology 142:127-134, 1982
20. Maggi G, Casadio C, Caballo A, Cianci R, Molinatti M, Ruffini E.
Thymoma: results of 241 operated cases. Ann Thorac Surg 51:152-9, 1991
21. Yagi K, Hirata T, Gukuse T, et al. Surgical treatment for invasive
thymoma, especially when the superior vena cava is invaded. Ann Thorac
Surg 61:521-524, 1996
22. Nakahara K, Ohno K, Hashimoto J, et al. Thymoma: results with
complete resection and adjuvant postoperative irradiation in 141
consecutive patients. J Thorac Cardiovasc Surg 95:1041-1047, 1988
23. Caruso ES, Vasallo BC, Beveraggi EJ, Dalurzo L. Quistes y tumores
del mediastino. Análisis de 100 observaciones. Rev Argen Ciruj. 1996 pp:
22 – 30
24. Gawrychowski J, Rokicki M, Gabriel A, et al. Thymoma: the usefulness
of some prognostic factors for diagnosis and surgical treatment. Eur J
of Surg Oncol 26:203-208, 2000
25. Adkins RB, Maples MD, Hainsworth JD. Primary malignant mediastinal tumors. Ann Thorac Surg 38(6):648-59, 1984
26. Okumura M, Miyoshi S, Yoshitaka F, et al. Clinical and functional
significance of WHO classification on human thymic epithelial neoplasms:
a study of 146 consecutive tumors. Am J Surg Pathol 25(1)103-110, 2001
27. Davis RD, Oldham HN, Sabiston DC. Primary cist and neoplasms of the
mediastinum: recent chages in clinical presentation, methods of
diagnosis, management and results. Ann Thorac Surgery 44:229, 1987
28. Wilkins EW Jr. Thymoma: surgical management, in Wood DE, Thomas CR
(eds): Mediatinal tumors: Update 1995; Medical Radiology-diagnostic
imaging and radiation oncology volume. Heidelberg, Germany,
Springer-Verlag, 1995, pp 19-25
29. Hejna M, Habert I, Raderer M. Nonsurgical management of malignant thymoma. Cancer 88(9):1871-1884, 1999
30. Suster S, Rosai J. Thymic carcinoma: a clinicopathologic study of 60 cases. Cancer 67:1025-1032, 1991
31. Lanfenfeld J, Graeber GM. Current management of thymoma. Surg Oncol Clin N Am 8(2):327-39, 1999
32. Chilosi M, Iannucci A, Menestrina F, Lestani M, Scarpa A, et al.
Immunohistochemidcal evidence of active thymocyte proliferation in
thymoma. Its possible role in the patho 27:70 – 76, 1996
33. Woo-IcK Y, Efird J, Quintanilla-Martinez L, Choi N, Harris N. cell
kinetic study of thymic epithelial tumors using PCNA (PC10) and Ki-67
(MIB-1) antibodies. Human Pathol 27:70 – 76, 1996
Related publications
- Angeles Medical Group, English Version
- Chemotherapy and side effects
- Clinical Trials
- CRECE english version
- Cáncer de cabeza y cuello experiencia en Guatemala con cetuximab
- Hepatocarcinoma en Guatemala
- Molecular zone, clinical laboratory
- Our services
- Slides and presentations
- Studies in Guatemala
- Types of cancer
- Video conferences
- Who we are